By Catherine Zandonella, Office of the Dean for Research
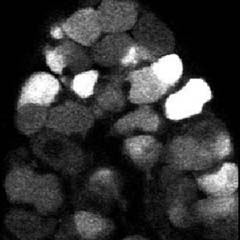
The pain and itching associated with shingles and herpes may be due to the virus causing a “short circuit” in the nerve cells that reach the skin, Princeton researchers have found.
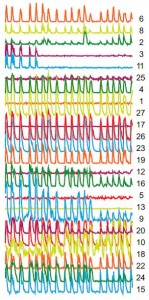
This short circuit appears to cause repetitive, synchronized firing of nerve cells, the researchers reported in the journal Proceedings of the National Academy of Sciences. This cyclical firing may be the cause of the persistent itching and pain that are symptoms of oral and genital herpes as well as shingles and chicken pox, according to the researchers.
These diseases are all caused by viruses of the herpes family. Understanding how these viruses cause discomfort could lead to better strategies for treating symptoms.
The team studied what happens when a herpes virus infects neurons. For research purposes the investigators used a member of the herpes family called pseudorabies virus. Previous research indicated that these viruses can drill tiny holes in neurons, which pass messages in the form of electrical signals along long conduits known as axons.
The researchers’ findings indicate that electrical current can leak through these holes, or fusion pores, and spread to nearby neurons that were similarly damaged, causing the neurons to fire all at once rather than as needed. The pores were likely created for the purpose of infecting new cells, the researchers said.
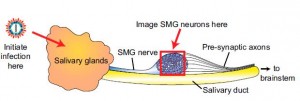
The investigators observed the cyclical firing of neurons in a region called the submandibular ganglia between the salivary glands and the brain in mice using a technique called 2-photon microscopy and dyes that flash brightly when neurons fire. (Movie of synchronized firing of herpes-infected neurons.)
The team found that two viral proteins appear to work together to cause the simultaneous firing, according to Andréa Granstedt, who received her Ph.D. in molecular biology at Princeton in 2013 and is the first author on the article. The team was led by Lynn Enquist, Princeton’s Henry L. Hillman Professor in Molecular Biology and a member of the Princeton Neuroscience Institute.
The first of these two proteins is called glycoprotein B, a fusion protein that drills the holes in the axon wall. A second protein, called Us9, acts as a shuttle that sends glycoprotein B into axons, according to the researchers. “The localization of glycoprotein B is crucial,” Granstedt said. “If glycoprotein B is present but not in the axons, the synchronized flashing won’t happen.”
The researchers succeeded in stopping the short circuit from occurring in engineered viruses that lacked the gene for either glycoprotein B or Us9. Such genetically altered viruses are important as research tools, Enquist said.
Finding a way to block the activity of the proteins could be a useful strategy for treating the pain and itching associated with herpes viral diseases, Enquist said. “If you could block fusion pore formation, you could stop the generation of the signal that is causing pain and discomfort,” he said.
Granstedt conducted the experiments with Jens-Bernhard Bosse, a postdoctoral research associate in molecular biology. Assistance with 2-photon microscopy was provided by Stephan Thiberge, director of the Bezos Center for Neural Circuit Dynamics at the Princeton Neuroscience Institute.
The team previously observed the synchronized firing in laboratory-grown neurons (PLoS Pathogens, 2009), but the new study expands on the previous work by observing the process in live mice and including the contribution of Us9, Granstedt said.
Shingles, which is caused by the virus herpes zoster and results in a painful rash, will afflict almost one out of three people in the United States over their lifetime. Genital herpes, which is caused by herpes simplex virus-2, affects about one out of six people ages 14 to 49 years in the United States, according the Centers for Disease Control and Prevention.
This research was funded by National Institutes of Health (NIH) Grants NS033506 and NS060699. The Imaging Core Facility at the Lewis-Sigler Institute is funded by NIH National Institute of General Medical Sciences Center Grant PM50 GM071508.
Granstedt, Andréa E., Jens B. Bosse, Stephan Y. Thiberge, and Lynn W. Enquist. 2013. In vivo imaging of alphaherpesvirus infection reveals synchronized activity dependent on axonal sorting of viral proteins. PNAS 2013 ; published ahead of print August 26, 2013, doi:10.1073/pnas.1311062110

You must be logged in to post a comment.